Import Licenses
Importing Medical Devices
India’s medical device industry was largely unregulated until the Medical Devices Rules, 2017 came into effect on January 1, 2018. Under this framework, the Central Drug Standard Control Organization (CDSCO) oversees licensing and compliance.
- Central Licensing Authority (CLA): Handles import licensing and Class C & D medical device manufacturing.
- State Licensing Authority (SLA): Manages Class A & B medical device manufacturing and wholesale licenses.
Foreign manufacturers with prior approvals from the U.S., EU, Canada, Japan, or Australia can apply for registration in India with a valid wholesale license holder.

Application Process Import Licenses
Product Evaluation
Documentation & Compliance
Appoint an Authorized Indian Agent
Application Submission & Review
Regulatory Follow-up & Approval
Post-Compliance Obligations
Once licensed, the manufacturer must adhere to:
✅ Presenting the license upon regulatory request.
✅ Reporting unexpected serious adverse events within 15 days.
✅ seeking prior approval for any significant modifications.
✅ Conducting pre-release lab tests as per Rule 83(3).
✅ Maintaining manufacturing & sales records for audit.
✅ Notifying authorities if production halts for over 30 days.
Accorpmed ensures smooth compliance with CDSCO regulations, making medical device registration hassle-free.
Import License (Form MD-15)
The Indian medical device industry relies heavily on imports to meet healthcare demands. To import medical devices, including in-vitro diagnostic devices, into India, an Import License (Form MD-15) is mandatory. This license is issued by the Central Drugs Standard Control Organization (CDSCO) under the Medical Device Rules, 2017.
CDSCO ensures that all imported medical devices meet quality and safety standards. The licensing process includes evaluation, inspection, and strict compliance with regulatory norms.

Documents Required for Import License (Form MD-15)
- Power of Attorney, authenticated in India by a Magistrate of First Class, the Indian Embassy, or equivalent authority through apostille.
- Valid Manufacturing License (Self-attested copy).
- Free Sale Certificate, duly apostilled/notarized, along with Marketing Authorization from the National Regulatory Authority of the country of origin.
- Latest Inspection/Audit Report by Notified Bodies or National Regulatory Authorities within the last three years.
- ISO 13485 Certificate (Notarized copy) of the actual manufacturer.
- Full Quality Assurance Certificate/CE Type Examination Certificate/CE Product Quality Assurance (Notarized copies).
- CE Design Certificate (Notarized copy).

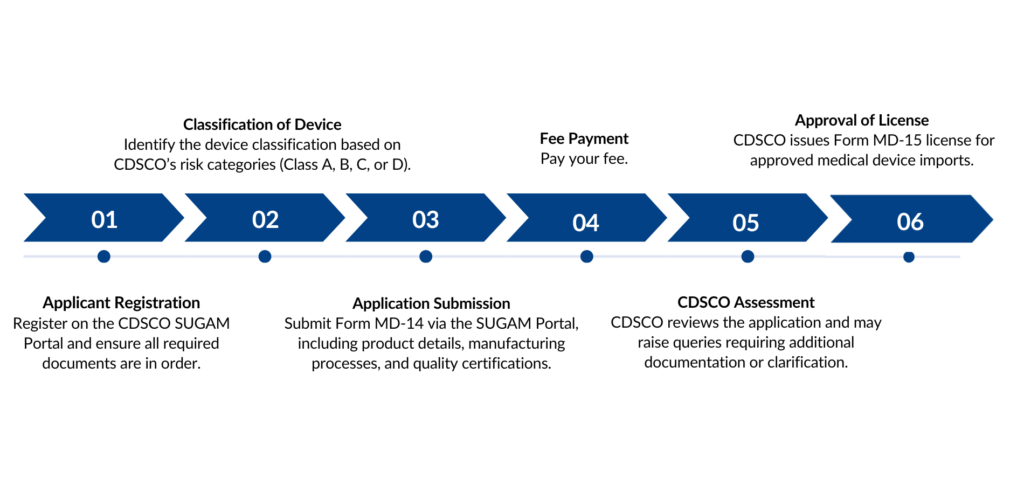
Application Process for MD 15 License
Contact Information
support@accorpmed.com
+91 99682 97717
909, ITL Twin Tower, B-9, Netaji Subhash Place, Pitampura, Delhi-110034 (INDIA)
2025-26 © accorpmed.com | All Rights Reserved




