Authorized EconomicOperator (AEO) Certification
The Authorized Economic Operator (AEO) Scheme is a program developed under the World Customs Organization (WCO) framework, specifically the SAFE Framework of Standards, aimed at securing and facilitating global trade. An AEO (Authorized Economic Operator) is a business entity engaged in the international movement of goods. This entity must comply with the national customs laws and is approved by the national administration, in accordance with the WCO or similar supply chain security standards.
Types of AEO Certification in India
AEO T1
Certification is granted based solely on document submission.
AEO T2
In addition to T1 compliance, both document verification and onsite inspection are conducted.
AEO T3
AEO T3 is for T2 holders with 2+ years; physical check if major changes or non-compliance.
AEO LO
For logistics operators, AEO requires document checks and onsite inspection.
Eligibilty of AEO registration in India
Financial Soundness
Demonstrate financial soundness and proper internal control systems.
International Engagement
Be involved in the international movement of goods (importers, exporters, logistics providers, etc.).
Minimum 1 Year of Business Experience
Comply with Customs laws and have been in business for at least one year.
No Ongoing Investigations or serious Infractions
Not be under investigation or have serious Customs violations.
Adequate Infrastructure
Have sufficient infrastructure to securely manage international shipments.
Authorized Representative
Appoint a local authorized representative (for foreign businesses).
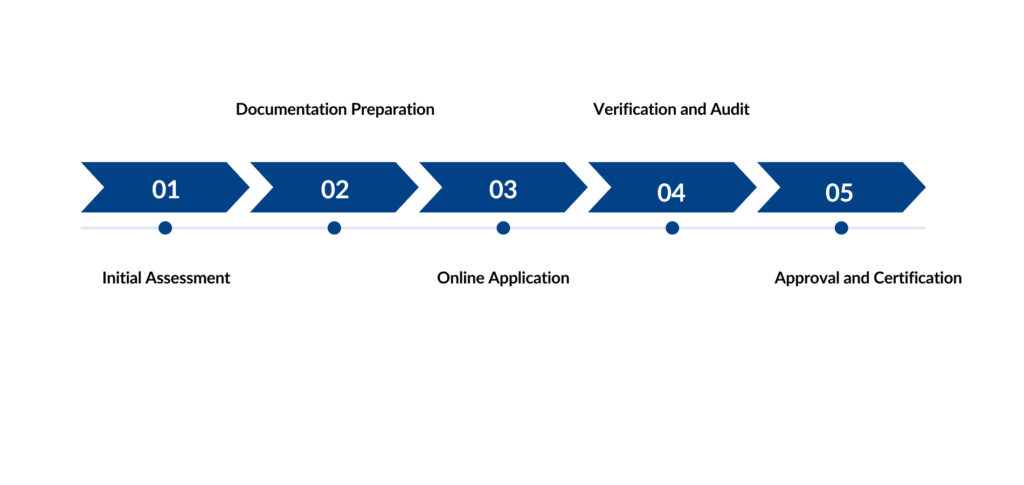
Process of AEO registration
FDA Registration(US Market)
The Authorized Economic Operator (AEO) Scheme is a program developed under the World Customs Organization (WCO) framework, specifically the SAFE Framework of Standards, aimed at securing and facilitating global trade. An AEO (Authorized Economic Operator) is a business entity engaged in the international movement of goods. This entity must comply with the national customs laws and is approved by the national administration, in accordance with the WCO or similar supply chain security standards.

Registration Process of FDA
Determine Device Classification Class I (Low Risk)
Minimal regulatory requirements. Class II (Moderate Risk) – May require 510(k) clearance. Class III (High Risk) – Requires Premarket Approval (PMA).
Establishment Registration
All manufacturers must register with the FDA and list their devices. Foreign manufacturers need a U.S. Agent.
Establishment Registration
All manufacturers must register with the FDA and list their devices. Foreign manufacturers need a U.S. Agent.
Compliance & Labeling
Adhere to FDA Quality System Regulations (QSR). Ensure proper labeling and UDI compliance (if applicable).
Post-Market Requirements
Medical Device Reporting (MDR) for adverse events. FDA inspections and periodic audits.
Documents Required for FDA Registration (US Market)
Airway Bill & Invoice Bill of Lading
Order for purchase Specific
Documentation for Each Commodity
List of Items to Consider
List of Growers
Labelling Copies
Documentation proving the identity of the product's true owner

CE Marking (EU Market)
The CE stands for Conformite Europeenne, which means European Conformity. The Conformité Européene (CE) Mark is defined as the European Union’s (EU) mandatory conformity marking for regulating the goods sold within the European Economic Area (EEA) since 1985

Registration Process for CE Marketing
Documents Required for CE Marking (EU Market)
- General description of the product.
- Conceptual design and manufacturing drawings, including component and circuit schemes if applicable.
- Descriptions and explanations to aid understanding of the drawings and schemes.
- List of harmonized and non-harmonized standards applied during the conformity assessment process.
- Test reports and risk assessment files.
- Copies of conformity documentation for critical product components.
- Instructions for use.
- Copy of the Declaration of Conformity.

Free Sale (Certificate)
A Free Sale Certificate (FSC), also known as a Certificate for Export, is an official document issued by regulatory authorities, certifying that a medical device is freely sold and complies with applicable regulations. This certificate is essential for exporting regulated or notified medical devices to international markets.

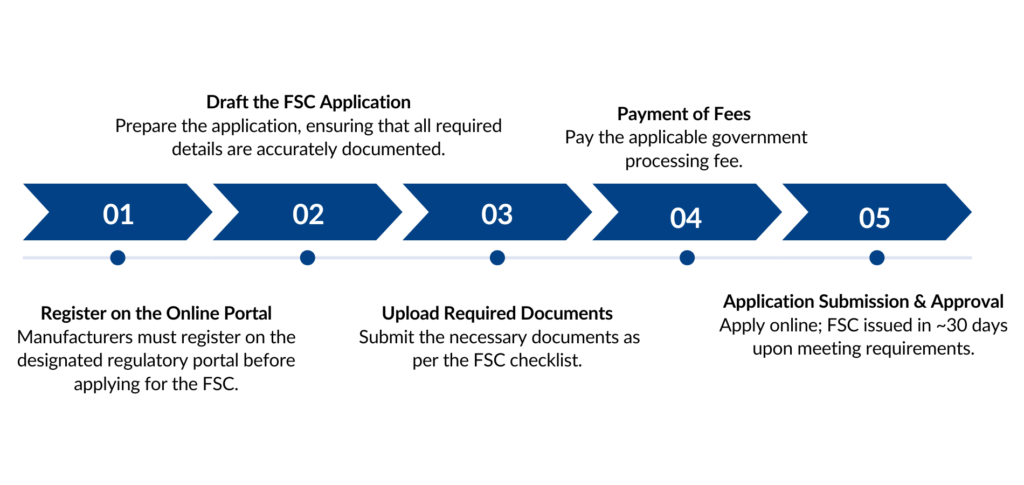
Application Process for Free sale certificate
Documents Required for Free Sale Certificate for Medical Devices
📌 Cover Letter – On company letterhead, signed and stamped by the organization’s head.
📌 Undertaking on Rs.100 Notarized Stamp Paper – Declaring no market complaints or adverse events.
📌 List of Products – A document listing all medical devices requiring the FSC.
📌 Manufacturing License & Approved Product List – Verifying compliance with regulatory standards.

Contact Information
support@accorpmed.com
+91 99682 97717
909, ITL Twin Tower, B-9, Netaji Subhash Place, Pitampura, Delhi-110034 (INDIA)
2025-26 © accorpmed.com | All Rights Reserved




