CDSCO
Obtaining a CDSCO license for medical device registration in India is a complex and time-consuming process. It requires extensive documentation and expertise in regulatory compliance.

Classification of MedicalDevices in India
Class A (Low Risk)
Bandages, surgical masks
Class B (Moderate Risk)
Syringes, nebulizers
Class C (High Risk)
Implantable devices, pacemakers
Class D (Highest Risk)
Heart valves, life-supporting equipment
Medical Device Registration Process
Classification & Documentation
Devices are categorized based on risk, and applicants must prepare technical documents, compliance certificates, and regulatory approvals.
Application Submission
The application, along with the required documents, is submitted via the CDSCO portal. Registration fees must be paid at this stage.
Review & Inspection
CDSCO evaluates the application, reviews documents, and may conduct facility inspections for quality assurance.
Approval & Licensing
Upon meeting all compliance requirements, CDSCO grants the necessary license for sale and distribution.
Manufacturing License Requirements
Obtaining a Manufacturing License for Class A & B devices (regulated by the State Licensing Authority) and Class C & D devices (regulated by the Central Licensing Authority).
Providing required documents such as a Site Master File, Device Master File, ISO 13485 certification, and manufacturing process details.
Registering on the CDSCO Sugam portal and submitting MD-3 & MD-7 forms for approval.
Form MD 5
Manufacturing medical devices require adherence to strict regulations set by the Central Drug Standard Control Organisation (CDSCO). At AccorpMed, we guide manufacturers through the licensing process for Class A and Class B medical devices, ensuring full compliance with regulatory requirements.

Form MD 5 Application Process
Form MD 9
The Central Drugs Standard Control Organisation (CDSCO) regulates the manufacturing of Class C & D medical devices in India. To manufacture, sell, or distribute these devices, companies must obtain a Form MD-9 license by applying through Form MD-7 under the Medical Device Rules, 2017.
Application Process MD 9

Test Licence
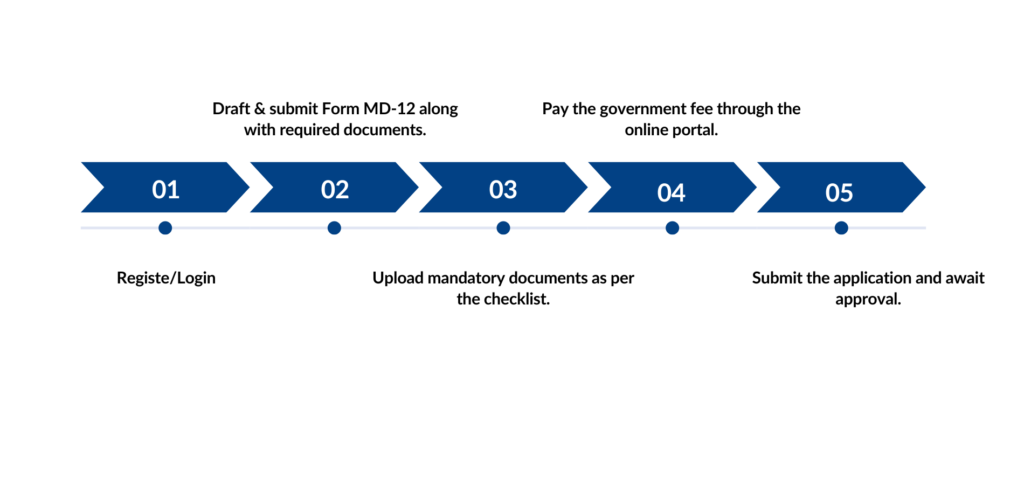
Manufacturing small quantities of Class A, B, C, or D medical devices for testing, evaluation, research, or training in India requires a Test License (Form MD-13) from CDSCO. To obtain this, applicants must submit Form MD-12 through the Ministry of Health and Family Welfare's Sugam portal.

Documents Required for Test Licence
- Medical device description (intended use, design, materials).
- List of qualified personnel.
- Quality certifications.
- Equipment details & premises schematic plan.

Application Process Test Licence
Contact Information
support@accorpmed.com
+91 99682 97717
909, ITL Twin Tower, B-9, Netaji Subhash Place, Pitampura, Delhi-110034 (INDIA)
2025-26 © accorpmed.com | All Rights Reserved




