BIS Certificationfor Medical Devices
The Bureau of Indian Standards (BIS) is India’s national regulatory body ensuring product safety, quality, and reliability. BIS certification is mandatory for certain medical devices and helps manufacturers gain consumer trust, comply with Indian regulations, and facilitate market entry. The certification process involves rigorous testing, factory inspections, and ongoing surveillance to maintain compliance with Indian standards (IS guidelines). BIS operates through multiple regional and branch laboratories across India, ensuring stringent quality control.
Accorpmed provides end-to-end support for BIS certification, including application filing, factory inspection readiness, compliance documentation, and liaising with regulatory authorities to streamline approval.

BIS Registration Process
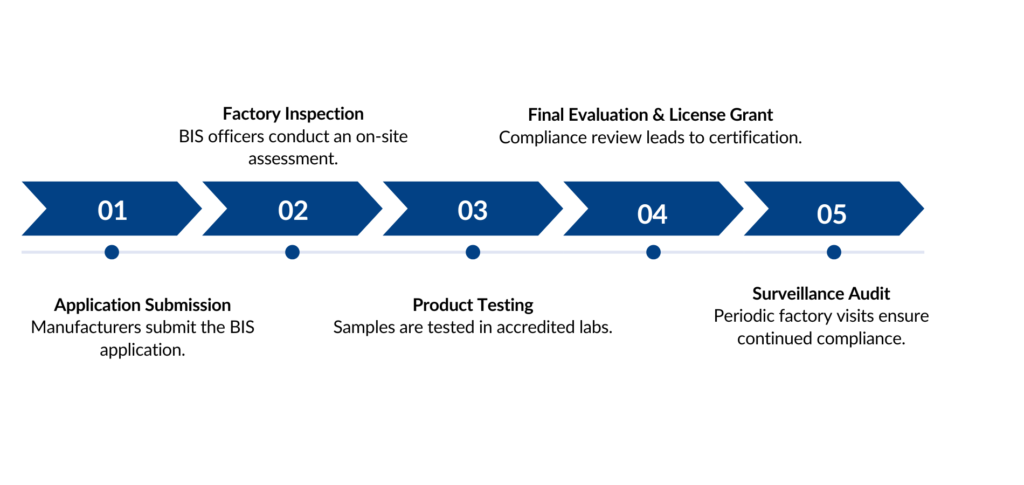
Required Documents for BIS
- Company incorporation proof
- Manufacturing process flow
- Machinery & equipment list
- Testing facility details
- Product test reports
- BIS authorization letter (if applicable)

Types of BIS Certification
Contact Information
support@accorpmed.com
+91 99682 97717
909, ITL Twin Tower, B-9, Netaji Subhash Place, Pitampura, Delhi-110034 (INDIA)
2025-26 © accorpmed.com | All Rights Reserved




